Pipeline
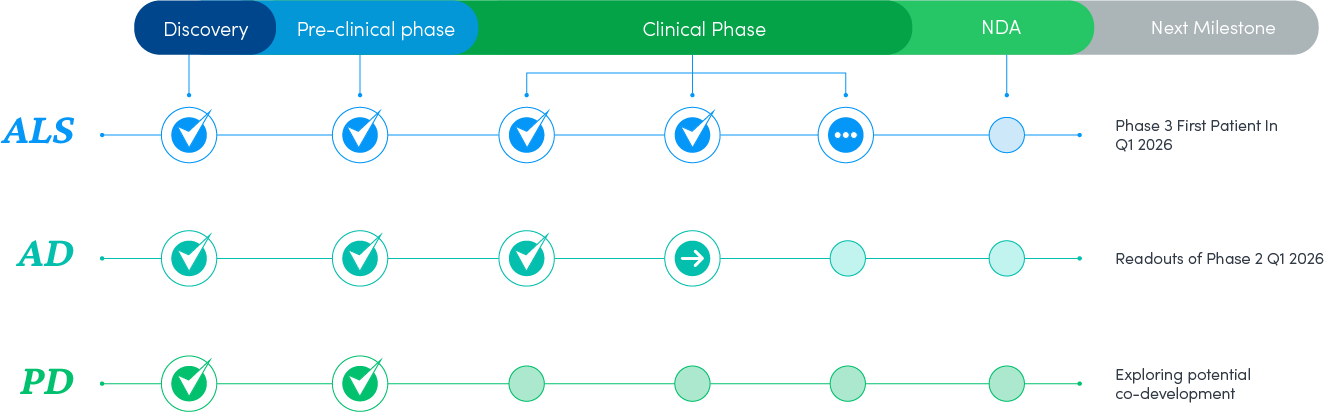
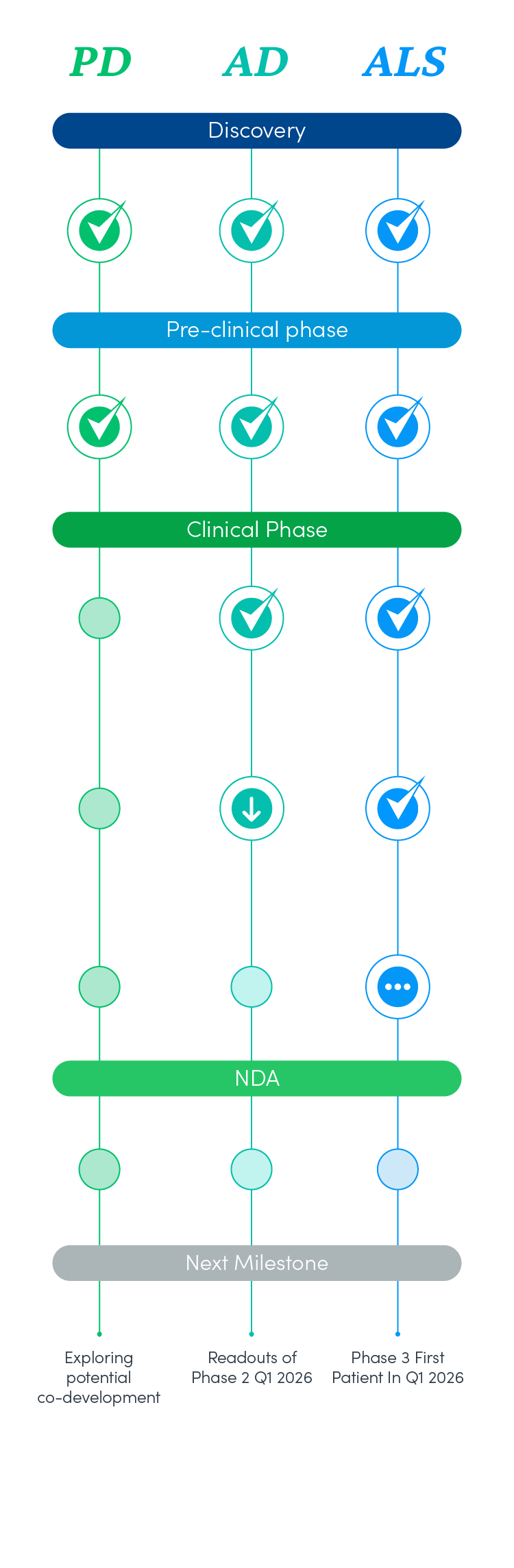
NeuroSense Pipeline
NeuroSense is developing novel therapies for neurodegenerative conditions by transforming breakthrough science into disease-modifying treatments.

PrimeC is a novel formulation composed of unique doses of two FDA-approved drugs, Ciprofloxacin and Celecoxib, which aim to synergistically inhibit the progression of ALS by aiming to regulate microRNA synthesis, reduce neuroinflammation, and influence iron accumulation.

Due to the many shared pathways between neurodegenerative diseases, the hypothesis is that a disease-modifying drug for one, can lay the foundations for effective drugs for other neurodegenerative diseases. Therefore, we are working to develop a drug for Alzheimer’s based on the foundations of PrimeC. We have initiated the pre-clinical stage, testing CogniC in in-vitro models representing Alzheimer’s disease pathologies, and now initiating a phase 2 double blind placebo controlled study with 20 AD patients.

There are many shared pathways between Parkinson’s disease and ALS, such as neuroinflammation, protein aggregation, mitophagy, excitotoxicity, oxidative stress, iron accumulation, and dysregulation of miRNAs. Therefore, we are working to develop a drug for Parkinson’s based on the foundations of our drug, PrimeC, for ALS. We have initiated the pre-clinical stage, testing StabiliC in in-vivo models of Parkinson’s, assessing morphological and functional effects, exploring potential co-development with collaborators that have core focus in Parkinsons.
Join Our Newsletter